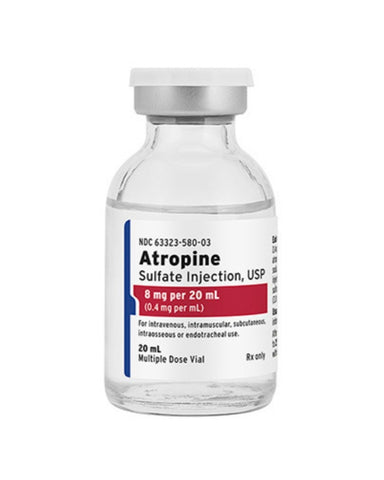
Fresenius Kabi Atropine Sulfate Injection, 8mg, 20mL Vial (ea)
Description
Atropine Sulfate Injection, USP (Vial)
Features:
- Strength: 8mg/20ml (0.4 mg/ml)
- Unit Size: 20ml Vial
- NDC #6332358003
Specifications:
- Inactive Ingredients: Sodium Chloride, Benzyl Alcohol, Water, Sulfuric Acid
- Strength: 8mg/20mL
- Color: Clear, Colorless
- Application Type: Anticholinergic
- Route of Administration: Intravenous, Intramuscular, Subcutaneous
- Packaging Type: Dose Vial
- pH Range: 3 to 4.5
- Concentration: 0.4mg/mL
- Life Stage: 20mL
- NDC: 6332358003
- Sterile: Sterile
- Formulation: Injection, Liquid
- Storage Requirements: Store at 20 to 25°C (68 to 77°F), excursions permitted to 15 to 30°C (59 to 86°F)
- Solubility: Easily Soluble in Cold and Hot Water
- Latex Free: Yes
- Uses: Atropine is Used as an Antidote for Pilocarpine, Physostigmine, Isoflurophate
- Active Ingredient: Atropine, Sulfate